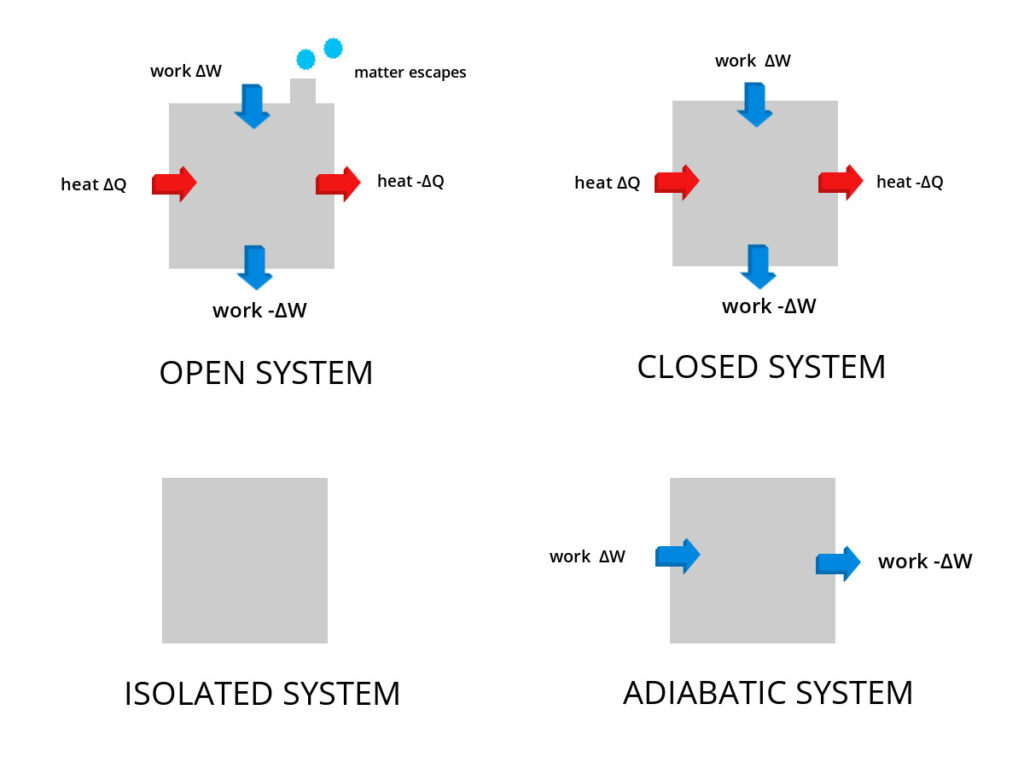
Imagine a world where nothing influences your environment. Isolated systems are fascinating examples of how energy and matter behave when cut off from external interactions. These systems maintain their properties without interference, making them crucial in fields like physics and thermodynamics.
Overview of Isolated Systems
Isolated systems play a crucial role in various scientific fields. They do not exchange energy or matter with their surroundings, allowing for controlled studies and experiments. Here are some notable examples:
- Thermos Flask: A thermos effectively maintains the temperature of its contents by minimizing heat transfer with the outside environment.
- Perfect Vacuum: In a perfect vacuum, no particles exist to interact with one another, creating an ideal isolated system for studying fundamental physical principles.
- Adiabatic Walls: These walls are designed to prevent heat transfer. When placed around a system, they ensure that no energy leaves or enters, making it isolated.
- Closed Containers: Sealed containers holding gases can act as isolated systems if external influences remain negligible.
Such examples illustrate how isolated systems facilitate understanding in physics and thermodynamics. By examining these systems, scientists can draw important conclusions about conservation laws and equilibrium states.
Characteristics of Isolated Systems
Isolated systems possess distinct characteristics that set them apart from other types of systems. They maintain their internal environment without interference from external forces. Understanding these traits enhances your knowledge of physical processes.
Energy Exchange
Isolated systems do not allow energy exchange with their surroundings. This characteristic enables the study of energy conservation principles in a controlled setting. For instance, when you place hot coffee in a thermos, it retains heat because the insulated walls prevent thermal energy from escaping. Another example is an adiabatic system where no heat flows across its boundary, allowing scientists to analyze changes in internal energy without external influence.
Matter Exchange
These systems also restrict matter exchange with the environment. As such, isolated systems provide a stable platform for experiments involving chemical reactions or physical changes. A sealed container acts as an isolated system if no gas or particles can enter or leave. For example, consider a gas-filled chamber equipped with tightly fitted seals; it remains unchanged despite external conditions, allowing accurate measurements over time.
Examples of Isolated Systems
Isolated systems provide excellent examples of how energy and matter behave without external influences. Here are some notable instances:
Ideal Gas in a Sealed Container
An Ideal Gas in a Sealed Container represents an isolated system effectively. In this scenario, gas particles move freely within the container while maintaining their total energy. Since there’s no exchange with the environment, properties like pressure and temperature remain constant unless altered internally.
Thermos Flask
A Thermos Flask serves as a practical example of an isolated system. It minimizes heat transfer between its contents and the external environment through vacuum insulation. This design keeps hot liquids hot and cold liquids cold for extended periods, illustrating energy conservation without outside interference.
The Universe
The universe itself can be considered an ultimate isolated system. While it’s constantly expanding, it doesn’t exchange matter or energy with anything beyond its boundaries. This concept allows scientists to study cosmic phenomena under conditions where outside influences are negligible, enabling deeper insights into fundamental laws of physics.
These examples highlight the significance of isolated systems in understanding physical processes and principles like conservation laws and equilibrium states.
Applications of Isolated Systems in Science
Isolated systems play a crucial role in various scientific fields, providing controlled environments for studying complex phenomena. Their unique characteristics allow you to analyze energy and matter without external interference.
Thermodynamics
In thermodynamics, isolated systems are essential for understanding energy conservation and transfer. An ideal example is the insulated thermos flask, which minimizes heat exchange with its surroundings. This design helps maintain temperature over time, illustrating how energy stays within the system. Additionally, adiabatic walls prevent heat loss or gain during experiments involving gases or liquids, ensuring accurate results when measuring changes in thermal energy.
Astrophysics
In astrophysics, isolated systems provide insights into cosmic events and structures. The universe itself acts as the ultimate isolated system, allowing scientists to study celestial bodies without outside influences. For instance, black holes represent regions where matter is confined so tightly that they create isolated environments affecting nearby stars and galaxies. Studying these phenomena helps you understand gravitational forces and the behavior of light under extreme conditions, expanding knowledge about space-time interactions.