Imagine standing in a room filled with various objects. Ever wonder what makes up everything around you? Identifying the items in the list below that are examples of matter is essential for understanding the physical world. Matter encompasses anything that has mass and occupies space, from the air you breathe to the chair you’re sitting on.
Understanding Matter
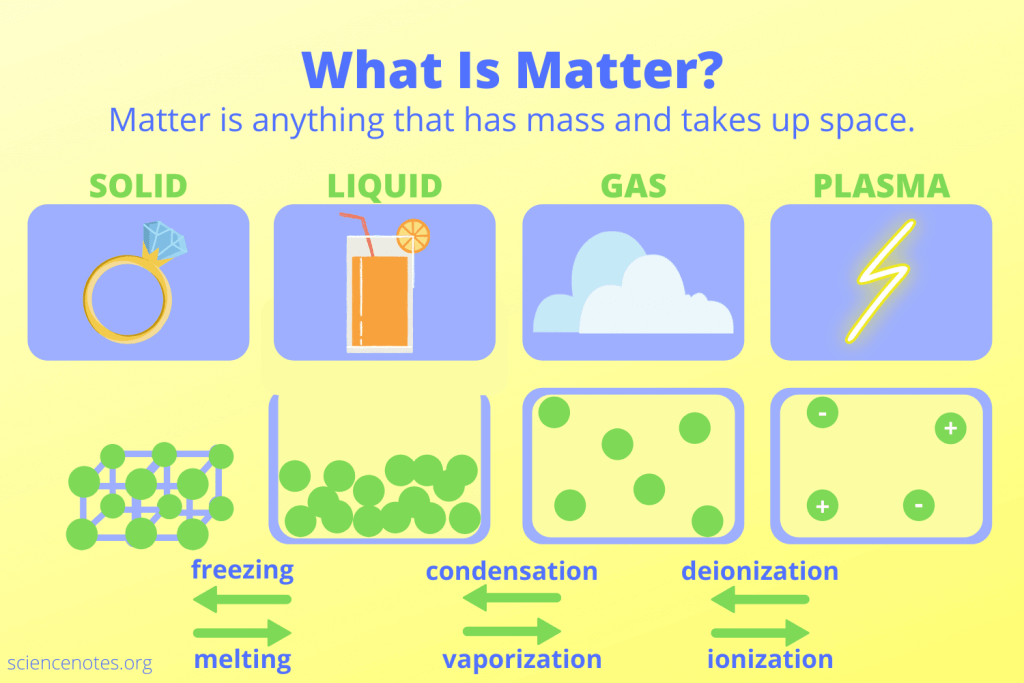
Matter includes everything that has mass and takes up space. You encounter matter in various forms every day, from solids like wood and metal to liquids such as water and juice. Gases, including oxygen and carbon dioxide, also qualify as matter.
Examples of solid matter include:
- Wood: Found in furniture or paper products.
- Metal: Present in coins, tools, and structures.
- Plastic: Used for containers and packaging.
On the liquid side, consider:
- Water: Essential for life; present in rivers, lakes, and your glass.
- Milk: Commonly consumed; found in dairy products.
- Oil: Used for cooking or lubrication.
Gaseous examples are less visible but equally important:
- Oxygen: Necessary for respiration; makes up about 21% of Earth’s atmosphere.
- Carbon Dioxide: Produced during respiration; crucial for plant photosynthesis.
Identifying these examples helps you understand the diverse nature of matter. By recognizing them around you, it becomes easier to grasp fundamental scientific concepts.
Types of Matter
Matter exists in different forms, each with unique characteristics. Understanding these types helps identify examples you encounter daily. Here’s a breakdown of the main types:
Solid
Solids have defined shapes and volumes. You can touch and feel solids easily. Common examples include:
- Wood: Found in furniture and construction.
- Metal: Used in tools, appliances, and vehicles.
- Plastic: Present in containers, toys, and packaging.
Each solid type exhibits distinct properties based on its composition.
Liquid
Liquids take the shape of their container while maintaining a fixed volume. You often interact with liquids without thinking about them. Examples include:
- Water: Essential for life; used for drinking and cleaning.
- Milk: Widely consumed as a beverage or ingredient.
- Oil: Utilized in cooking and lubrication.
These liquids flow freely but resist compression.
Gas
Gases fill available space completely without a fixed shape or volume. They’re all around you yet often go unnoticed. Key examples are:
- Oxygen: Vital for respiration; present in the atmosphere.
- Carbon Dioxide: Produced during respiration; critical for photosynthesis.
Gases expand to fit their environment, making them unique among matter types.
Examples of Matter
Identifying examples of matter enhances your understanding of the physical world. Matter includes various substances you encounter daily, categorized into everyday and scientific examples.
Everyday Examples
Water in a glass is matter. It occupies space and has mass. A wooden chair also qualifies as matter. It retains shape and volume. Other everyday items include:
- Metal utensils: These are solid materials with defined shapes.
- Plastic bottles: They hold liquids while maintaining form.
- Air in a room: Although invisible, it fills space and is essential for life.
Each item demonstrates different characteristics of matter that you experience regularly.
Scientific Examples
In scientific contexts, identifying matter becomes more precise. An atom represents the fundamental unit of matter. Atoms combine to form molecules, which make up everything around you. Additional examples include:
- Hydrogen gas (H₂): A key element that exists as a gas under standard conditions.
- Ice: Solid water that showcases a definite shape and volume.
- Carbon dioxide (CO₂): A gas produced by respiration; it’s vital for plant life.
These scientific instances reveal the complexity and variety within the concept of matter, enhancing your comprehension of its principles.
Processes Involving Matter
Matter undergoes various processes that change its state or composition. Understanding these processes helps you identify examples of matter in your daily life.
Physical Changes
Physical changes involve alterations in the form or appearance of matter without changing its chemical composition. Common examples include:
- Melting ice into water
- Dissolving sugar in coffee
- Breaking a glass into pieces
These changes are reversible, meaning you can return to the original state by reversing the process.
Chemical Changes
Chemical changes result in new substances being formed, altering the chemical properties of matter. Examples encompass:
- Burning wood, which produces ash and smoke
- Rust forming on iron when exposed to moisture
- Baking a cake, where ingredients transform into something new
These changes are often irreversible, indicating a permanent alteration of the original materials.