When it comes to understanding cell biology, grasping the concepts of hypertonic vs hypotonic solutions is crucial. Have you ever wondered how these terms affect the cells in your body? These two types of solutions play a significant role in processes like osmosis and fluid balance, impacting everything from hydration to nutrient absorption.
Overview of Hypertonic and Hypotonic Solutions
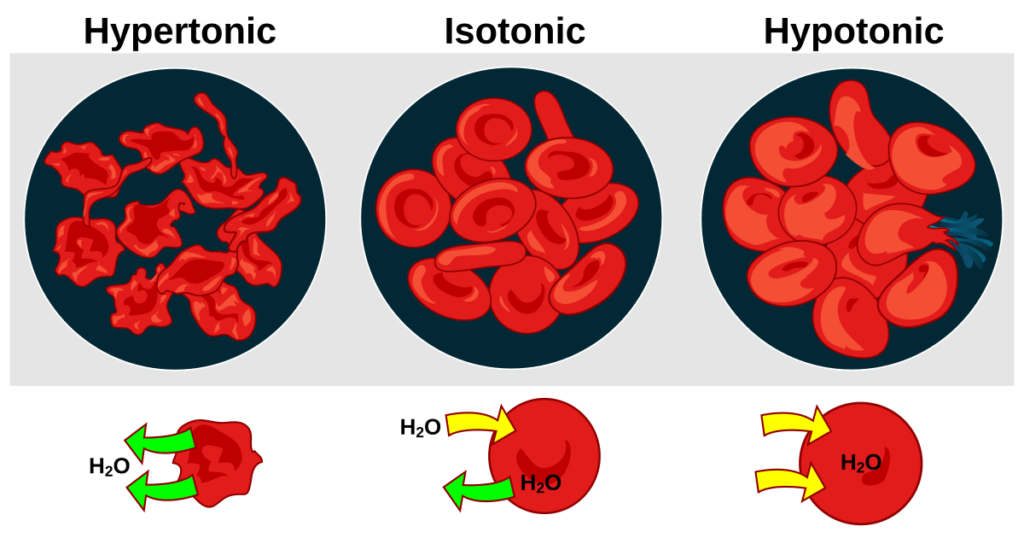
Hypertonic and hypotonic solutions play significant roles in cell biology. A hypertonic solution contains a higher concentration of solutes compared to the inside of a cell, leading to water moving out of the cell. This process causes cells to shrink or crenate. For instance, when red blood cells are placed in a hypertonic saline solution, they lose water and appear shriveled.
On the other hand, a hypotonic solution has a lower concentration of solutes than the inside of a cell, resulting in water entering the cell. This influx can cause cells to swell and potentially burst. An example includes placing red blood cells in distilled water; they take in excess water and may undergo hemolysis.
Understanding these concepts is essential for various applications such as medical treatments or laboratory experiments. You often encounter hypertonic solutions in IV fluids designed for specific conditions, while hypotonic solutions might be used for rehydration therapies after dehydration events.
In cellular environments, both types influence osmotic pressure significantly. They directly affect how nutrients are absorbed and waste products are expelled from cells. Knowing how these solutions work helps you better grasp their impact on hydration levels within your body or during scientific studies.
Properties of Hypertonic Solutions
Hypertonic solutions possess distinct properties that significantly impact cellular behavior. These solutions have a higher concentration of solutes compared to the fluid inside a cell, leading to crucial effects on osmosis and hydration.
Definition and Characteristics
A hypertonic solution is defined as one that contains more solutes than the cytoplasm of a cell. This characteristic results in water moving out of the cell through osmosis. When cells are exposed to hypertonic environments, they experience cell shrinkage due to the loss of water. This process can disrupt normal cellular functions and balance.
Examples of Hypertonic Solutions
Common examples include:
- Hypertonic saline: Often used in medical settings, it contains 3% or more sodium chloride.
- Dextrose solutions: A 5% dextrose solution can be hypertonic when mixed with certain fluids.
- Mannitol: Frequently utilized in clinical practice for its osmotic properties.
- Sea water: With an average salinity around 3.5%, it’s considered hypertonic compared to human cells.
Understanding these examples helps you appreciate how hypertonic solutions function within biological systems and their applications in medicine and research.
Properties of Hypotonic Solutions
Hypotonic solutions possess unique characteristics that differentiate them from hypertonic solutions. Understanding these properties is vital in various biological and medical contexts.
Definition and Characteristics
A hypotonic solution has a lower concentration of solutes compared to the inside of a cell. This difference causes water to flow into the cell through osmosis, leading to cellular swelling. Cells may become turgid as they take in water, which can result in bursting if the influx is excessive. The osmotic pressure balance plays a crucial role in maintaining cellular integrity.
Examples of Hypotonic Solutions
Several common examples illustrate hypotonic solutions:
- Distilled Water: Often used in laboratories, it’s pure with no dissolved substances.
- 0.45% Saline Solution: Frequently employed in medical settings for hydration purposes.
- Dextrose 2.5% Solution: Used for patients who require low levels of glucose.
- Tap Water: Safe for consumption but hypotonic relative to human cells.
Each example demonstrates how hypotonic solutions can influence cellular function and health significantly.
Comparison of Hypertonic vs Hypotonic
Understanding hypertonic and hypotonic solutions is crucial in cell biology. Both types of solutions significantly impact cellular behavior and function.
Effects on Cells
In a hypertonic solution, cells lose water, leading to shrinkage. For instance, if you place red blood cells in hypertonic saline, they shrivel as water moves out. This process can disrupt normal cellular functions.
Conversely, a hypotonic solution causes cells to swell as water flows into them. When red blood cells are placed in distilled water, they may burst due to excessive water intake. Maintaining osmotic balance is vital for cell integrity and overall health.
Practical Applications
Hypertonic and hypotonic solutions serve various practical purposes:
Hypertonic Solutions:
- Used in medical treatments for edema.
- Employed in laboratory settings for studying osmosis.
- Applied in fluid replacement therapies.
- Utilized in IV fluids to hydrate patients effectively.
These applications illustrate the significance of understanding these solutions and their effects on cells during medical interventions and scientific research.