Imagine a world where your favorite ice cream melts just a bit slower on a hot summer day. This fascinating phenomenon is known as freezing point depression, and it plays a crucial role in our everyday lives. By understanding how certain substances lower the freezing point of liquids, you can discover why salt is sprinkled on icy roads or why antifreeze is essential for your car’s engine.
Understanding Freezing Point Depression
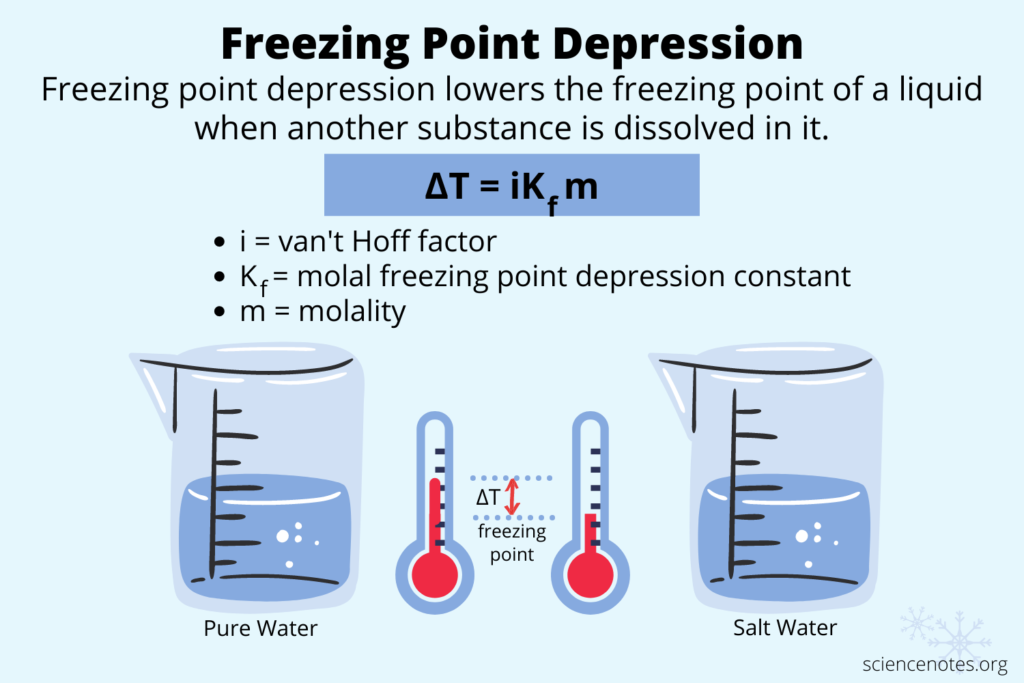
Freezing point depression occurs when certain substances are added to a liquid, lowering its freezing point. This concept plays a critical role in various scientific and practical applications.
Definition of Freezing Point Depression
Freezing point depression refers to the decrease in the temperature at which a liquid freezes due to the addition of solutes. For example, when salt dissolves in water, it disrupts the formation of ice crystals, resulting in a lower freezing point. The formula that describes this phenomenon is:
[ Delta T_f = K_f cdot m ]
where ( Delta T_f ) is the change in freezing point, ( K_f ) is the cryoscopic constant of the solvent, and ( m ) is the molality of the solution.
Importance in Chemistry
Freezing point depression serves as an essential principle in chemistry. It helps scientists understand colligative properties—those that depend on particle concentration rather than identity. Here are some important aspects:
- Real-world applications: In food science, using salt on icy roads prevents ice formation during winter.
- Antifreeze solutions: Ethylene glycol lowers water’s freezing point in car engines.
- Biological relevance: It maintains proper cellular function by preventing ice crystal formation within organisms.
Understanding these examples clarifies why freezing point depression matters not just theoretically but also practically.
Factors Affecting Freezing Point Depression
Several factors influence freezing point depression. Understanding these can help you grasp how different substances interact with solvents.
Solute Concentration
The concentration of solute directly impacts the extent of freezing point depression. As you increase the amount of solute, the freezing point decreases more significantly. For example:
- 1 mole of salt (NaCl) in 1 liter of water can lower the freezing point by about 3 degrees Celsius.
- 2 moles of sugar (C12H22O11) in 1 liter of water can result in a similar decrease, depending on its molecular weight.
More concentrated solutions lead to greater reductions in freezing points, demonstrating this relationship clearly.
Nature of the Solute
The chemical properties of a solute also play a crucial role. Ionic compounds typically cause greater freezing point depression than non-ionic compounds due to their dissociation into multiple particles. Consider these examples:
- Sodium chloride (NaCl) dissociates into two ions (Na+ and Cl-) when dissolved, enhancing its effect on lowering the freezing point.
- Conversely, glucose (C6H12O6) doesn’t dissociate; it remains as one particle per molecule, resulting in less pronounced effects on freezing point depression.
Different solutes exhibit varying capacities to disrupt solvent structures, leading to unique outcomes for each substance used.
Applications of Freezing Point Depression
Freezing point depression plays a crucial role in various practical applications. Understanding these uses provides insight into everyday phenomena and industrial processes.
Real-World Examples
You see freezing point depression at work in your daily life. For example, using salt on icy roads lowers the freezing point of water, preventing ice formation and enhancing safety during winter months. Similarly, antifreeze solutions are vital for car engines; they prevent coolant from freezing in cold temperatures, ensuring optimal performance. Additionally, ice cream manufacturers utilize this phenomenon to maintain creamy textures by lowering the freezing point of their mixtures.
Industrial Applications
Industrially, freezing point depression serves multiple purposes. In food preservation, adding salt or sugar to foods slows down freezing rates and enhances texture retention. Furthermore, in pharmaceuticals, certain formulations use solutes that lower the freezing points of liquid medications for safe transport and storage. Also important is its role in cryogenics; scientists rely on it to keep substances at extremely low temperatures for experimentation and storage purposes.
| Application | Example |
|---|---|
| Road Safety | Salt prevents ice formation on roads |
| Automotive | Antifreeze prevents engine coolant from freezing |
| Food Preservation | Sugar slows down the freeze rate in products |
| Pharmaceuticals | Solutes ensure liquid medications remain stable |
| Cryogenics | Maintains low temperatures for experiments |
Experimentation and Measurement Techniques
Experimenting with freezing point depression involves precise methods to observe changes in freezing points due to solute addition. Understanding these techniques enhances comprehension of the phenomenon and its applications.
Laboratory Methods
Common laboratory techniques include:
- Cryoscopic Method: This method measures the change in freezing point as a solute dissolves in a solvent. By recording the temperature at which a solution freezes, you can calculate the extent of freezing point depression.
- Thermal Analysis: Differential scanning calorimetry (DSC) allows for detailed thermal profiles when cooling solutions. It provides accurate measurements of phase changes and is useful for examining various substances.
- Refractometry: While primarily used to measure concentration, refractometry also indicates how solutes affect freezing points by analyzing their impact on light refraction.
These methods provide reliable data on how different substances influence freezing behavior.
Data Analysis
- Collecting Temperature Data: Record temperatures precisely during experiments to observe when phase changes occur.
- Calculating Freezing Point Depression: Use formulas like (Delta T_f = i cdot K_f cdot m), where (i) represents van ‘t Hoff factor, (K_f) denotes the cryoscopic constant, and (m) is molality.
- Comparing Results: Analyze results from various trials under consistent conditions. This helps identify patterns or anomalies linked to specific solutes.
Strong data analysis supports your understanding of colligative properties and their real-world implications.