In the world of clinical trials, source documents play a crucial role in ensuring data integrity and compliance. But what exactly are these documents, and why do they matter? From patient consent forms to laboratory results, source documents provide the foundational evidence needed for accurate reporting and regulatory approval.
As you delve into this topic, you’ll discover various examples of source documents that not only support trial findings but also uphold ethical standards. Each document serves a specific purpose in documenting participant interactions and outcomes. Are you curious about how these examples can impact the overall success of clinical research? Understanding their significance is essential for anyone involved in the field. Let’s explore some key examples that highlight their importance in clinical trials.
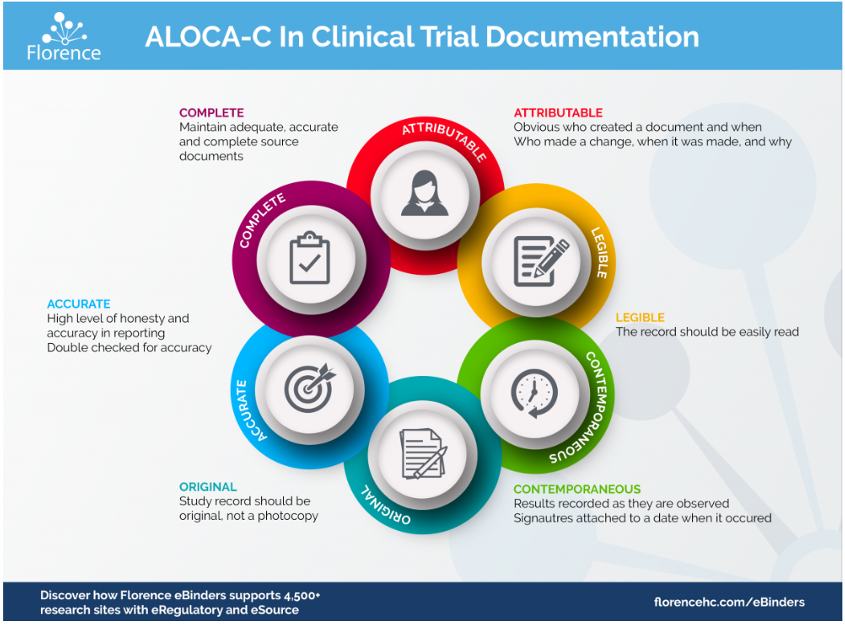
Importance Of Source Documents In Clinical Trials
Source documents play a crucial role in clinical trials. They ensure the accuracy and reliability of data collected during research. Understanding their importance helps you appreciate how they support regulatory compliance and ethical standards.
- Patient consent forms: These confirm that participants understand the trial and agree to participate.
- Case report forms (CRFs): These capture key information about each participant’s health status and treatment.
- Laboratory results: These provide objective evidence of a participant’s condition before, during, and after the trial.
- Medical history records: These offer context regarding a participant’s background, which can affect outcomes.
Each document serves as evidence of what occurred during the study. You can’t underestimate their value for audits or inspections by regulatory bodies. Furthermore, accurate source documents facilitate transparent reporting of findings.
Ultimately, robust source documentation promotes trust in clinical research outcomes. When you think about it, these documents are vital for protecting patient rights while ensuring high-quality data collection.
Types Of Source Documents
Source documents play a vital role in clinical trials, ensuring data accuracy and compliance. Here are key examples of source documents used in this context.
Case Report Forms
Case Report Forms (CRFs) collect trial-related data directly from each participant. These forms include demographic information, medical history, and treatment details. Researchers use CRFs to track participant progress and outcomes. They help standardize data collection across sites, ensuring consistent reporting. Each CRF must align with the study protocol for regulatory approval.
Patient Medical Records
Patient Medical Records provide comprehensive health histories that inform clinical trial designs. These records include diagnoses, past treatments, and laboratory results essential for understanding participant backgrounds. Accessing accurate medical records aids eligibility assessments and helps monitor patient safety during trials. Additionally, they serve as a reference for adverse event reporting.
Investigation Brochures
Investigation Brochures contain critical information about the investigational product. This document outlines the drug’s pharmacology, potential risks, and benefits associated with participation in the trial. It’s crucial for informing both investigators and participants about what to expect throughout the study process. Keeping this brochure up-to-date ensures compliance with regulatory requirements.
Informed Consent Forms
Informed Consent Forms ensure that participants understand their rights before joining a trial. These documents detail study procedures, risks involved, and confidentiality assurances. Participants sign these forms to confirm their voluntary agreement to participate based on adequate knowledge of the study’s goals. Properly executed informed consent promotes ethical standards in clinical research while protecting patient autonomy.
Examples Of Source Documents In Clinical Trials
Source documents play a vital role in clinical trials, providing essential evidence for data integrity and compliance. Here are some key examples:
Clinical Trial Protocols
Clinical trial protocols outline the study design, objectives, and methodology. These documents detail how the trial will be conducted, including participant eligibility criteria, treatment regimens, and endpoints. You’ll find that adherence to these protocols ensures consistency across sites and helps mitigate risks during the trial.
Lab Reports
Lab reports present objective data regarding test results related to the investigational product. These reports include blood tests, imaging studies, or other diagnostic evaluations that inform researchers about participants’ health status. They provide critical insights into safety and efficacy during the trial.
Adverse Event Reports
Adverse event reports document any unexpected medical occurrences experienced by participants. These reports capture details about symptoms, severity levels, timing of events, and relationships to the investigational product. Regulatory bodies require this documentation to assess safety risks associated with new treatments.
Monitoring Visit Reports
Monitoring visit reports summarize findings from site visits conducted by monitors. During these visits, monitors evaluate compliance with the protocol and regulatory requirements. The reports also highlight any discrepancies or corrective actions needed to ensure data accuracy throughout the study.
These examples illustrate how source documents contribute significantly to maintaining high standards in clinical research while ensuring participant safety.
Best Practices For Managing Source Documents
Maintaining organized and accurate source documents is vital in clinical trials. Start by establishing a clear filing system. You can use both digital and physical formats, but ensure everything is easily accessible for audits.
Standardize document templates across all sites. This facilitates uniformity in data collection, making it easier to compare information later. Using consistent terminology reduces confusion among team members.
Implement regular training sessions. Keeping everyone updated on best practices ensures that your staff understands the importance of these documents. Training should cover handling procedures, data entry protocols, and compliance requirements.
Audit your source documents periodically. Regular reviews help identify discrepancies or missing information early. Consider conducting internal audits every six months to maintain high standards.
Create a backup system for digital files. Data loss can occur unexpectedly; therefore, having backups protects against potential issues. Use cloud storage solutions with encryption to secure sensitive patient data.
By following these best practices, you contribute significantly to the integrity of clinical trial outcomes while prioritizing participant safety and regulatory compliance.