Esters are fascinating compounds that play a crucial role in both nature and industry. Have you ever wondered what gives fruits their delightful aromas or why certain oils have unique scents? Esters are the answer, providing those distinct flavors and fragrances. These organic compounds not only enhance our culinary experiences but also find applications in everything from perfumes to plastics.
Overview of Esters
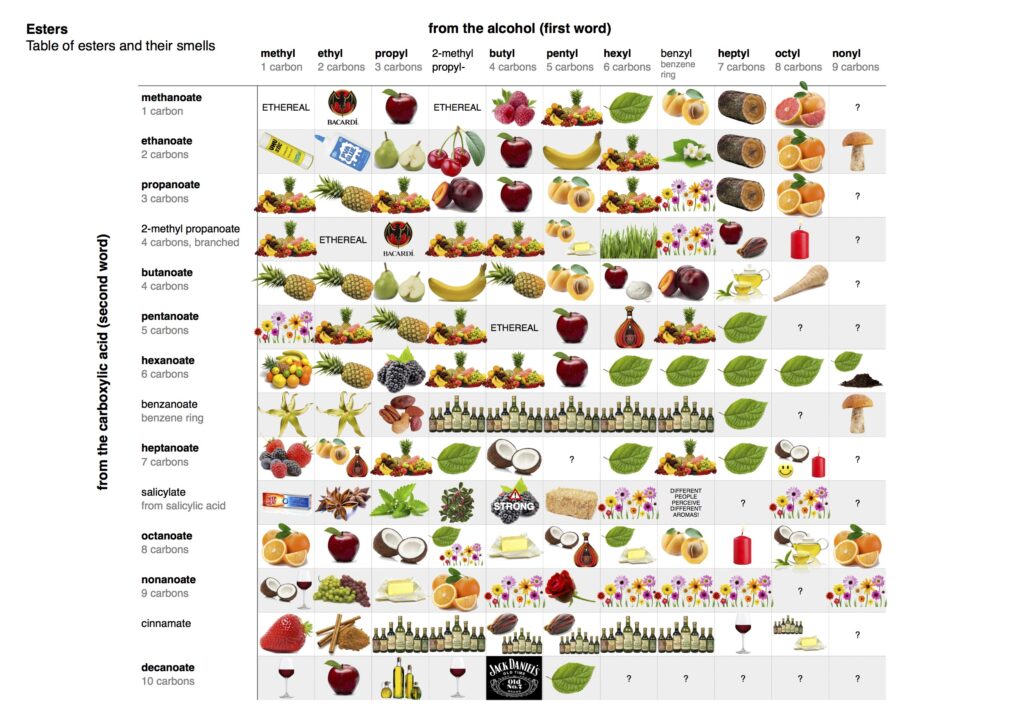
Esters are organic compounds formed from the reaction between an alcohol and a carboxylic acid. They play a significant role in both nature and various industries, contributing to flavors, fragrances, and materials.
Definition of Esters
Esters are characterized by their functional group, which consists of a carbonyl group (C=O) adjacent to an ether link (–O–). This structure gives esters their unique properties. For example, ethyl acetate, commonly used as a solvent in nail polish removers, is an ester derived from ethanol and acetic acid.
Importance in Chemistry
In chemistry, esters serve as key intermediates in numerous reactions. They participate in processes like esterification—where acids react with alcohols—and transesterification—important for biodiesel production. Additionally, esters can influence the physical properties of substances such as boiling points and solubility. For instance:
- Methyl butanoate contributes fruity aromas.
- Butyl acetate acts as an effective paint solvent.
Understanding esters expands your grasp on organic chemistry’s applications across different fields.
Types of Esters
Esters can be categorized into two main types: simple esters and complex esters. Each type has distinct characteristics and applications.
Simple Esters
Simple esters are formed from the reaction of a single alcohol and a single carboxylic acid. These esters typically have straightforward structures, making them easier to identify and study. Examples include:
- Ethyl acetate: Commonly used as a solvent in nail polish removers.
- Methyl acetate: Found in various industrial applications due to its effective solvent properties.
- Butyl acetate: Often utilized in coatings and adhesives for its excellent solubility.
These compounds usually exhibit pleasant fruity aromas, contributing to their popularity in the food industry.
Complex Esters
Complex esters arise when multiple alcohols or acids react, often leading to larger molecules with more intricate structures. They can provide unique flavors or fragrances. Examples include:
- Polyester resins: Used in textiles, bottles, and films for their durability.
- Triglycerides: Found in fats and oils; they consist of glycerol bonded to three fatty acids.
- Phosphoesters: Essential in biochemistry for energy transfer within cells via ATP.
These compounds play significant roles across many industries, from food production to materials science.
Properties of Esters
Esters exhibit unique properties that make them significant in various applications. Understanding these properties provides insights into their behavior and uses.
Physical Properties
Esters are generally characterized by their pleasant odors, which often resemble the scents of fruits or flowers. They typically have low boiling points compared to other organic compounds due to weaker intermolecular forces. For instance:
- Ethyl acetate has a boiling point of 77 °C, making it effective as a solvent.
- Butyl acetate, with its fruity aroma, boils at 126 °C.
- Methyl acetate is known for its low viscosity and boiling point of 57 °C.
These physical attributes contribute to esters’ roles in fragrances and food flavorings.
Chemical Properties
Chemically, esters are reactive compounds. They undergo hydrolysis reactions when mixed with water, breaking down into an acid and an alcohol. This reaction can be catalyzed by either heat or acid. Key examples include:
- In the presence of strong acids like sulfuric acid, ethyl acetate can hydrolyze to form acetic acid and ethanol.
- Transesterification involves exchanging the alkoxy group of an ester with another alcohol; this process is crucial in biodiesel production.
Moreover, esters participate in condensation reactions where they can combine with various reactants to create more complex molecules.
Applications of Esters
Esters play a crucial role across various industries and biological systems. Their unique properties make them valuable in multiple applications.
Industrial Uses
Esters find widespread use in the industrial sector. For example, ethyl acetate serves as a popular solvent in paint and coatings. Additionally, butyl acetate is often utilized in nail polish removers due to its effective dissolving power. Other notable examples include:
- Methyl acetate: Commonly used as a solvent in adhesives.
- Propylene glycol esters: Employed as emulsifiers in food products.
- Polyester resins: Found in textiles and plastics, offering durability and flexibility.
These compounds enhance product performance while providing desirable characteristics like low volatility and pleasant aromas.
Biological Significance
In biological systems, esters also hold significant importance. They are integral components of fats and oils, known as triglycerides. These molecules store energy efficiently within living organisms. Furthermore, certain esters contribute to flavor profiles in fruits, enhancing sensory experiences during consumption.
You might find it interesting that phosphoesters are vital for cellular processes involving energy transfer through ATP (adenosine triphosphate). This illustrates how esters support essential functions at the molecular level.
Overall, from their industrial versatility to their biological roles, esters significantly impact both everyday life and complex biochemical processes.