Have you ever wondered how everything around you is categorized? The classification of matter plays a crucial role in understanding the world we live in. From the air we breathe to the water we drink, all substances can be grouped based on their properties and behaviors.
In this article, you’ll explore various examples that illustrate how matter is classified into solids, liquids, gases, and more. You’ll discover how these categories help scientists and everyday people alike make sense of complex materials. By diving into the characteristics of each classification, you’ll gain insight into why certain substances behave as they do.
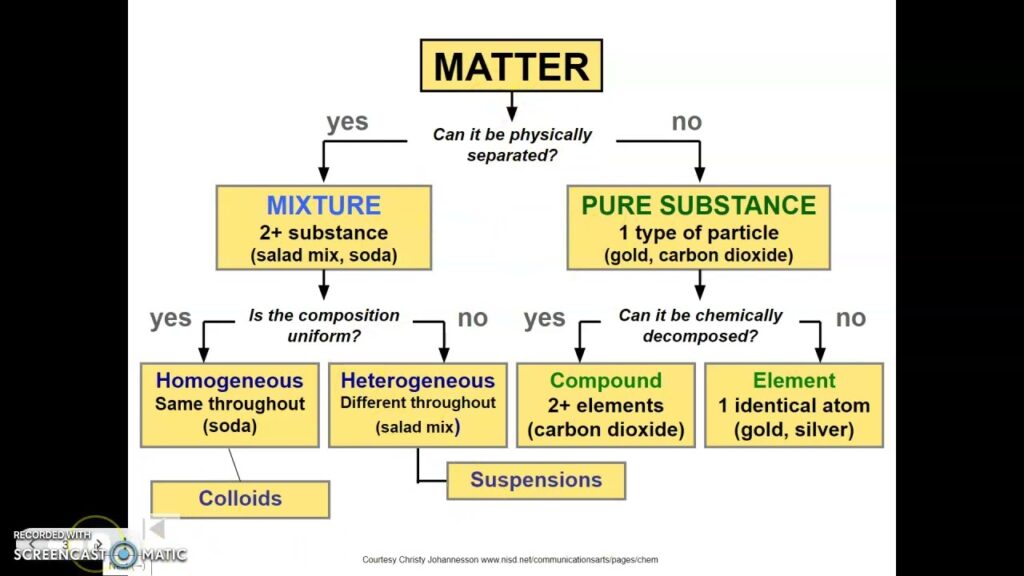
Overview Of Classification Of Matter
Classifying matter helps you understand various substances and their interactions. This classification covers solids, liquids, gases, and more specialized categories like mixtures and compounds.
Importance Of Classifying Matter
Classifying matter simplifies the study of chemistry. By grouping substances based on common properties, you gain insights into their behaviors. For example, knowing that metals conduct electricity allows for better understanding in electrical applications. Furthermore, classifying matter aids in identifying unknown substances quickly. It also plays a crucial role in fields like pharmacology and environmental science.
Historical Development
The classification of matter has evolved significantly over time. Ancient Greek philosophers proposed early theories about elements like earth, water, air, and fire. Later developments by scientists like John Dalton introduced atomic theory in the early 1800s. In the mid-19th century, Dmitri Mendeleev created the periodic table, organizing elements based on atomic weight and properties. Modern classifications now include extensive data on molecular structure and behavior.
Types Of Matter
Matter can be categorized into several distinct types, each with unique characteristics. Understanding these classifications helps you grasp how different substances interact and behave in various environments.
Pure Substances
Pure substances consist of only one type of particle. They maintain consistent properties throughout and can be classified as elements or compounds.
- Elements: These are the simplest forms of matter that cannot be broken down further by chemical means. Examples include:
- Hydrogen (H)
- Oxygen (O)
- Carbon (C)
- Compounds: Compounds are made up of two or more elements chemically bonded together. Notable examples include:
- Water (H₂O)
- Sodium chloride (NaCl), commonly known as table salt
- Glucose (C₆H₁₂O₆)
Each pure substance showcases specific physical and chemical properties, making them essential for scientific study and practical applications.
Mixtures
Mixtures contain two or more different substances that retain their individual properties. They may be homogeneous or heterogeneous.
- Homogeneous mixtures: These appear uniform throughout. Common examples include:
- Saltwater
- Air
- Vinegar
- Heterogeneous mixtures: These show distinct components that can often be visually identified. Examples include:
- Salad
- Oil and water
- Sand mixed with iron filings
Understanding mixtures is crucial because they exhibit varied behaviors depending on their composition and interactions among components.
States Of Matter
Matter exists primarily in three states: solid, liquid, and gas. Each state displays different physical characteristics based on temperature and pressure changes.
- Solids maintain a fixed shape and volume due to tightly packed particles.
- Liquids take the shape of their container while maintaining a definite volume with loosely arranged particles.
- Gases, however, fill any available space as particles are far apart with minimal attraction between them.
Recognizing these states aids you in predicting how matter will behave under changing conditions.
Mixtures
Mixtures consist of two or more substances combined while retaining their individual properties. They can be categorized into homogeneous and heterogeneous mixtures, each with distinct characteristics.
Homogeneous Mixtures
Homogeneous mixtures appear uniform throughout. You can’t easily distinguish the different components. Common examples include:
- Saltwater: When salt dissolves in water, it forms a solution that looks the same everywhere.
- Air: A mixture of gases like nitrogen and oxygen, which is consistent regardless of where you sample it.
- Vinegar: A solution of acetic acid in water that remains evenly mixed.
These mixtures exhibit similar properties across all samples, making them essential in various applications such as cooking and chemistry.
Heterogeneous Mixtures
Heterogeneous mixtures have visibly different components. You can easily identify and separate the substances within them. Examples include:
- Salad: Composed of various ingredients like lettuce, tomatoes, and cucumbers that maintain their unique textures and flavors.
- Oil and Water: These two liquids do not mix; oil floats on top due to differences in density.
- Concrete: Made from aggregates like sand, gravel, and cement; its structure varies throughout.
You’ll notice that these mixtures present varied physical appearances and behaviors depending on how they are combined. Understanding these differences is crucial for practical applications in both science and everyday life.
Characteristics Of Different Matter Classes
Understanding the characteristics of different classes of matter enhances your comprehension of the material world. Each class exhibits unique features that define how they interact and behave.
Physical Properties
Physical properties refer to observable traits without changing the substance’s chemical composition. Common examples include:
- Color: The color of substances like copper (reddish-brown) or sulfur (yellow).
- Melting Point: Ice melts at 0°C while iron melts at 1,538°C.
- Density: Gold has a high density compared to aluminum.
- Solubility: Salt dissolves in water, while sand does not.
These properties help identify and differentiate substances, making them crucial for practical applications in various fields.
Chemical Properties
Chemical properties describe how a substance interacts with other materials during chemical reactions. Examples include:
- Reactivity with Acid: Metals like magnesium react vigorously with hydrochloric acid.
- Flammability: Gasoline ignites easily, whereas water doesn’t burn.
- Oxidation States: Iron can oxidize to form rust when exposed to moisture and air.
Recognizing these properties is vital for predicting how substances will behave under specific conditions, especially in industrial and laboratory settings.
Applications Of Classification Of Matter
Classifying matter plays a crucial role in various fields, influencing how you interact with substances daily. This method provides clear insights into the properties and behaviors of materials, leading to practical applications across multiple disciplines.
In Chemistry
In chemistry, classification helps identify and predict how substances react under certain conditions. For example:
- Acids and Bases: Knowing whether a substance is an acid or base guides you in understanding its reactivity with other chemicals.
- Organic vs. Inorganic Compounds: This distinction aids in determining the appropriate methods for synthesis or analysis.
- Solubility Classifications: Identifying solutes based on their solubility informs decisions about solutions used in experiments.
Understanding these classifications enhances your ability to perform safe and efficient experiments.
In Industry
Industry relies heavily on classifying matter for production and quality control. Here are some examples:
- Material Selection: Engineers classify metals as ferrous or non-ferrous when choosing materials for construction projects.
- Pharmaceuticals: Drug development requires categorizing compounds as active ingredients or excipients to ensure efficacy and safety.
- Food Processing: Classifying food items as perishable or non-perishable helps manage inventory effectively.