Have you ever wondered how elements transform into different ones? Beta decay is a fascinating process that illustrates this transformation in action. It’s not just a theoretical concept; it plays a crucial role in the stability of atomic nuclei. In this article, you’ll discover various beta decay examples that highlight its significance in both nature and technology.
Understanding Beta Decay
Beta decay plays a crucial role in the transformation of elements and atomic stability. This process involves the emission of beta particles from unstable atomic nuclei, leading to changes in both mass and charge.
What Is Beta Decay?
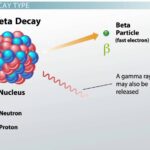
Beta decay is a type of radioactive decay where an unstable nucleus transforms into a more stable one. During this process, either a neutron converts into a proton or vice versa. The emitted particle can be either an electron (beta-minus) or a positron (beta-plus). This transformation modifies the element’s identity while releasing energy in the form of radiation.
Types of Beta Decay
There are two primary types of beta decay:
- Beta-minus (β-) decay: In this type, a neutron is transformed into a proton, emitting an electron and an antineutrino. For example, carbon-14 undergoes beta-minus decay to become nitrogen-14.
- Beta-plus (β+) decay: Here, a proton changes into a neutron while emitting a positron and neutrino. An example includes fluorine-18 decaying into oxygen-18 through beta-plus emission.
Both types serve as essential processes for stabilizing isotopes and happen naturally over varying timescales.
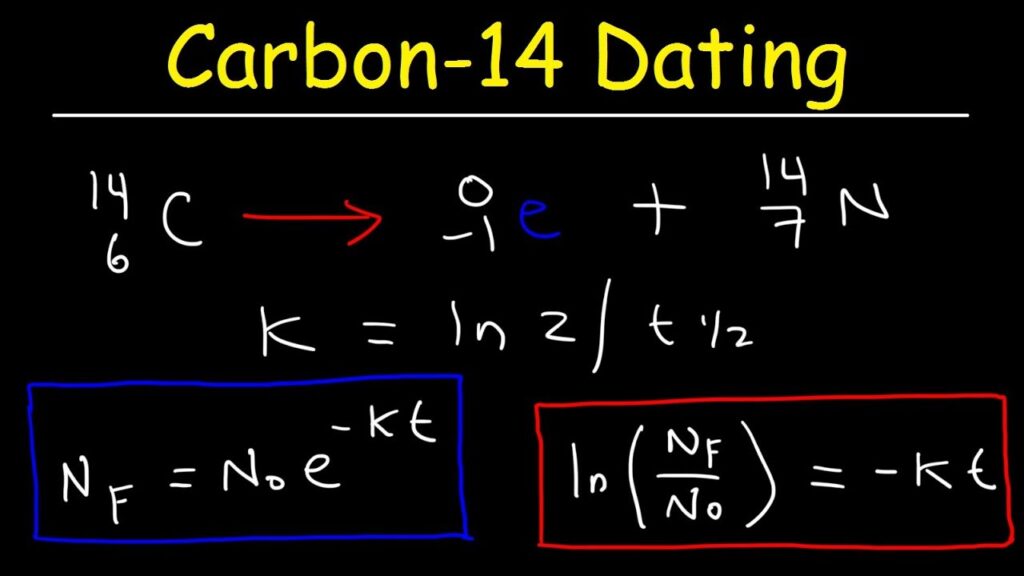
Beta Decay Example: Carbon-14
Carbon-14 serves as a key example of beta decay in action. This isotope, with an atomic mass of 14, undergoes beta-minus decay, transforming into nitrogen-14. During this process, a neutron in the carbon nucleus converts into a proton, releasing a beta particle (electron) and an antineutrino.
Mechanism of Carbon-14 Decay
In beta-minus decay, the mechanism begins when Carbon-14’s unstable nucleus triggers the conversion of one neutron to a proton. As this change occurs:
- Beta Particle Emission: An electron is emitted as a beta particle.
- Antineutrino Release: An antineutrino is also released.
- Nitrogen Formation: The result is nitrogen-14, which has one more proton than carbon-14.
This transformation not only stabilizes the nucleus but also illustrates how elements can change through nuclear processes.
Significance of Carbon-14 in Dating
Carbon-14 plays an essential role in radiocarbon dating. This method allows scientists to determine the age of organic materials accurately by measuring their remaining Carbon-14 content. Here’s why it matters:
By understanding how much Carbon-14 remains in a sample compared to its initial levels, researchers can infer its age effectively.
Implications of Beta Decay
Beta decay has significant implications across various fields, particularly in medicine and nuclear physics. Understanding these implications helps you grasp the broader impacts of this radioactive process.
Applications in Medicine
Beta decay plays a pivotal role in medical diagnostics and treatment. For instance, certain isotopes undergo beta decay to produce radiation that targets cancer cells. This targeted approach minimizes damage to surrounding healthy tissue while maximizing tumor destruction. Some examples include:
- Iodine-131: Used for treating thyroid cancer.
- Phosphorus-32: Applied in treating blood disorders like polycythemia vera.
These applications underline the importance of beta decay in developing effective therapies that improve patient outcomes.
Role in Nuclear Physics
In nuclear physics, beta decay is essential for understanding elemental transformations and stability within atomic structures. It provides insights into how unstable isotopes evolve over time through specific processes, such as:
- Nuclear reactions: These involve changes within atomic nuclei that lead to new elements.
- Neutrino studies: Researchers explore neutrinos generated during beta decay to test fundamental theories about particle interactions.
Thus, studying beta decay enhances your comprehension of both atomic behavior and the underlying principles governing nuclear interactions.
Common Misconceptions About Beta Decay
Many misconceptions surround beta decay that can lead to confusion.
One common myth is that beta decay only involves electrons. In reality, it includes both beta-minus decay, which emits electrons, and beta-plus decay, which emits positrons. Understanding these two types clarifies the processes involved.
Another misunderstanding is the belief that all isotopes undergo beta decay. Not every unstable isotope experiences this type of decay; some may undergo alpha or gamma decay instead. Recognizing the specific conditions for beta decay helps in grasping its role in nuclear stability.
A prevalent misconception is that beta particles are harmless. While they are less damaging than alpha particles, they still pose risks at certain exposure levels. Awareness of proper safety measures when dealing with radioactive materials remains essential.
People often think beta decay occurs spontaneously without any external influence. Although it typically happens naturally over time, external factors like temperature and pressure can influence the rate of radioactive decay. This highlights how environmental conditions impact nuclear processes.
Understanding these misconceptions enhances your grasp of beta decay and its implications across various fields.